Abstract
Introduction: Without prompt treatment, heparin-induced thrombocytopenia (HIT) is associated with a daily rate of life- and limb-threatening thrombosis of 6.1%. Current recommendations are to empirically treat a patient with an intermediate or high pre-test likelihood of HIT with a non-heparin (alternative) anticoagulant while awaiting HIT laboratory test results. The real-world bleeding rates of empiric treatment with an alternative anticoagulant in patients with suspected HIT have not been well-described. We assessed the rates of major bleeding in a prospective cohort of patients with suspected HIT. Because HIT is generally considered a prothrombotic and not a prohemorrhagic disorder, we hypothesized that major bleeding would be less common in alternative anticoagulant-treated patients with HIT than in treated patients who were ultimately determined not to have HIT.
Methods: From June 2012 to January 2015, we enrolled 310 patients with suspected HIT at the Hospital of the University of Pennsylvania and an affiliated community hospital. All patients underwent HIT laboratory testing with a poly-specific PF4/heparin ELISA and in-house serotonin release assay. Patients were adjudicated to be HIT-positive or HIT-negative by an independent adjudication panel of HIT experts based on clinical course and results of HIT laboratory testing. We retrospectively reviewed the electronic health record to assess for the presence of a major bleeding event from the time of suspicion of HIT until hospital discharge or death. Major bleeding events were defined by International Society on Thrombosis and Haemostasis (ISTH) criteria (fall in hemoglobin ≥2 g/dL, transfusion of ≥2 units of packed red blood cells, fatal bleed, bleed occurring in a critical organ and/or requiring a repeat surgical intervention). Secondary outcomes of progressive or new thrombosis following suspicion of HIT testing, in-hospital and 30-day mortality were also assessed. Patients were considered to be treated for suspicion for HIT if they received an alternative anticoagulant following a HIT laboratory test order. The demographics and clinical features of patients defined by adjudicated HIT status were compared using the Wilcoxon test and chi-square tests for continuous and for categorical variables, respectively. The proportion of bleeding and thrombotic events were compared between groups using a chi-square test. All analyses were performed using Stata v14.2.
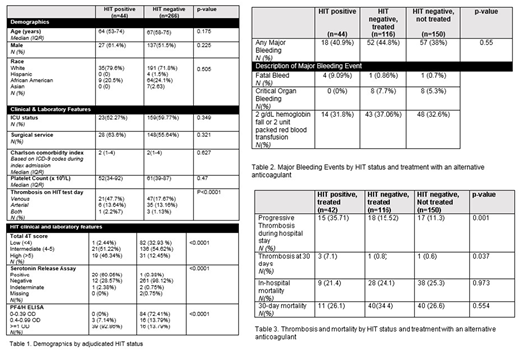
Results:The demographics of our cohort by adjudicated HIT status are displayed in Table 1. 44 patients in the cohort were adjudicated to be HIT-positive and 266 were adjudicated HIT-negative. 42/44 (95.5%) HIT-positive patients and 116 of 266 (43.6%) HIT-negative patients received an alternative anticoagulant.
Of those who were empirically treated with an alternative anticoagulant, the median duration of treatment was 3 days (IQR 2-5.5) in HIT-negative patients, compared with 11.5 days (IQR 6-16) in the HIT-positive group (p=0.001). The majority of treated patients received argatroban (88.0% of HIT-positive patients and 76.7% of HIT-negative patients). In those patients who received an alternative anticoagulant, a major bleeding event occurred in 40.9% of HIT positive patients and 44.8% HIT negative patients (p=0.45).The majority of major bleeding events met ISTH criteria based on hemoglobin fall or blood transfusion (Table 2). New or progressive thrombosis following suspicion for HIT was more common in HIT-positive patients (35.7%) than in patients who were HIT-negative and treated (15.5%) or not treated (11.3%) (p=0.001) (Table3).
Conclusions:
Contrary to our hypothesis, HIT was not protective against bleeding. A similar proportion of HIT-positive patients had ISTH major bleeding compared with HIT-negative patients treated with an alternative anticoagulant. The current paradigm for empiric treatment of patients with suspected HIT may need to be reconsidered in light of our findings of greater than expected bleeding associated with treatment. Limitations of our study include relatively small size, recruitment from a single health system, and predominant use of a single alternative anticoagulant (argatroban). Further studies are needed to determine whether our results apply to other centers and other alternative anticoagulants.
Pishko:Novo Nordisk: Research Funding. Cuker:Synergy: Consultancy; Genzyme: Consultancy; Kedrion: Membership on an entity's Board of Directors or advisory committees; Spark Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal